欢迎访问本课题组!
课题组的主要研究方向包括:
1)纳米材料化学;
2)表界面电化学;
3)能源电化学;
包括基于锂/钠离子电池、锂硫电池、锂氧气电池、超级电容器、燃料电池等电化学储能体系的研究。
课题组承担国家级新型器件开发项目、国家“973”计划课题、国家“863”计划项目、国家自然科学基金重点项目、省重点项目、厦门市重大专项等项目的研究,并与国内外同行开展合作研究。在国际重要期刊包括Nat. Comm., Chem, Energy Environ. Sci., J. Am. Chem. Soc., Angew. Chem. Int. Ed., Adv. Mater., Adv. Energy Mater., ACS nano等学术期刊上发表论文200余篇,获得国家发明专利30余件。近年来,结合多种原位与非原位表征技术,课题组致力于高比能、高功率电化学储能系统及关键储能材料的研究。具体涉及:
锂/钠离子电池
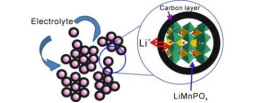
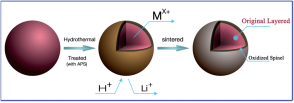
当前锂/钠离子电池面临的最重要的技术挑战是成本、耐用性和行驶里程,这在很大程度上取决于正极材料的性能。本课题组致力于对低成本、长循环、高比能锂/钠离子电池正极材料的研究。通过对正极材料的合成机理及充放电机理的研究,利用形貌结构调控及化学改性等策略,实现高倍率,长循环的优异电池性能。
锂硫电池
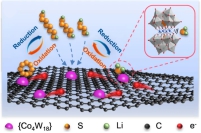
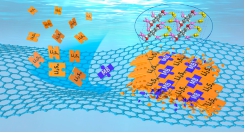
锂硫电池以其低成本、高比能(2600 Wh Kg-1)及环境友好等特性,成为最具发展潜力的二次电池储能体系之一。但活性物质的低电导率,以及多硫化锂的穿梭效应等问题却限制着其商业化的发展。本课题组致力于锂硫电池复合正极电催化及微观界面化学过程调控的研究。通过引入具有吸附、催化、键合等性质的功能性组分,以达到抑制穿梭和改善电池性能的目的。

锂氧气电池
锂氧气电池以金属锂为负极,空气中的氧气作为正极的活性物质,基于过氧化锂的生成与分解实现能量的存储与转化,具有极高的理论能量密度(3500 Wh kg-1)。本课题组致力于氧气电极固相表面电催化及溶液相催化的研究。通过对锂氧气电池充放电反应机理的研究,并结合电极结构方面的问题,构筑了有利于氧气发生反应的仿生开放式结构电极,以及设计开发了新型高效的溶液相催化剂。


金属阳极保护
锂、钠金属阳极的比容量分别为3861 mAh g-1和1165 mAh g-1,远高于离子电池阳极的石墨或硬质合金。但长时间循环过程中不稳定的沉积/剥离是金属电池性能缺失的一大关键问题。实现稳定和安全的金属阳极对于这两种电池系统将具有潜在的应用价值。本课题组致力于集流体设计、电解质的改性等方面的研究,以实现抑制枝晶生长、改善电极/电解质界面稳定性,促进金属阳极长期稳定的沉积/剥离过程。
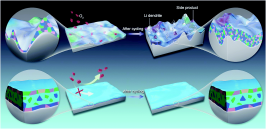
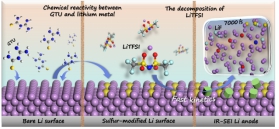
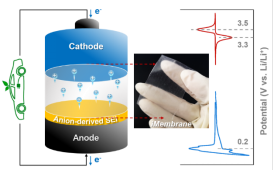
固态电解质等高安全电解质体系
高安全性是锂二次电池实际应用的首要条件。本课题组致力于研发多种创新型高安全电解质体系,包括MOFs基准固态电解质、聚合物固态电解质、离子液体凝胶电解质、高浓电解液、阻燃电解液等,以构筑高比能高安全锂二次电池。系统研究电解质体相物化性质、电极/电解质界面性质、锂离子传输机制与电池性能之间的构效关系,并利用绝热加速量热法(ARC)、差速扫描量热法(DSC)以及穿刺实验等评估电池安全性。

Our group is mainly engaged in the chemistry of nanomaterials, surface/interface electrochemistry and energy electrochemistry based on lithium/sodium ion batteries, lithium sulfur batteries, lithium oxygen batteries, supercapacitors, fuel cells and other electrochemical energy storage systems. In addition, our group has presided over the research of key projects, national "973" program, national "863" program, national Natural Science Foundation of China key projects, provincial key projects, major special projects of Xiamen and other projects, and carried out cooperative research with domestic and foreign counterparts. Over 200 peer reviewed research articles has been published in internationally important journals including Nat. Comm., Chem, Energy Environ. Sci., J. Am. Chem. Soc., Angew. Chem. Int. Ed., Adv. Mater., Adv. Energy Mater., ACS Nano and other academic journals, and more than 30 national patents have been obtained. In recent years, combined with a variety of in-situ and non-in-situ characterization techniques, we mainly investigate the high specific energy and high power electrochemical energy storage systems and key energy storage materials.
Lithium/sodium ion batteries
The most important technical challenges facing lithium/sodium batteries today are cost, durability and range, which largely depend on the performance of cathode material. Our research group focuses on the cathode materials of low cost, long cycle and high specific energy lithium/sodium ion batteries. By studying the synthesis mechanism and charge-discharge mechanism of the cathode material, the excellent battery performance with high rate and long cycle was realized by using the strategy of morphology and structure regulation and chemical modification.
Lithium sulfur batteries
Lithium-sulfur battery has become one of the most promising secondary battery storage systems due to its low cost, high specific energy (2600 Wh kg-1) and environmental friendliness. However, the low electrical conductivity of the active substance and the shuttle effect of lithium polysulfide limit its commercial development. Our research group is devoted to the electrocatalysis and microinterface chemical process regulation of lithium-sulfur battery composite anode. The functional components with adsorption, catalytic and bonding properties were introduced to inhibit shuttle and improve the performance of Lithium-sulfur battery.
Lithium oxygen batteries
Lithium oxygen battery uses lithium metal as the negative electrode and oxygen in the air as the active substance of the positive electrode. Based on the generation and decomposition of lithium peroxide, it realizes energy storage and transformation, and has a very high theoretical energy density (3500 Wh kg-1). Our research group is devoted to the study of oxygen electrode solid-phase surface electrocatalysis and solution phase catalysis. Based on the study of the charging and discharging reaction mechanism of lithium oxygen battery and the problems of electrode structure, some bionic open structure electrode were constructed which were beneficial to the oxygen reaction, and some efficient solution phase catalysts were designed and developed.
Metal anode protection
The specific capacities of lithium and sodium metal anodes are 3861 mAh g-1 and 1165 mAh g-1, respectively, which are much higher than those of graphite or carbide anodes of ion batteries. However, unstable deposition/stripping during the long cycle is a key problem in the performance degradation of metal batteries. Metal anodes that achieve stability and safety will have potential applications for both battery systems. Our research group is committed to the research of current collectors design and electrolyte modification, so as to inhibit dendrite growth, improve the stability of electrode/electrolyte interface, and promote the long-term stable deposition/stripping process of metal anode.
Solid electrolyte and other high safety electrolyte system
High safety is the primary condition of lithium secondary battery application. Our group has committed to develop a variety of innovative high safety electrolyte systems, including MOFs quasi-solid electrolyte, polymer solid electrolyte, ionic liquid gel electrolyte, high concentration electrolyte, flame retardant electrolyte, to build high specific energy and high safety lithium secondary battery. We have systematically studied the physical and chemical properties of solid electrolyte, electrode/electrolyte interface properties, lithium-ion transport mechanism and battery performance. The safety of battery is also evaluated by ARC, DSC and puncture experiments.
地址:福建省厦门市思明区厦门大学固体表面物理化学国家重点实验室,厦门大学化学化工学院化学系,361005,中国福建省厦门市
Address: State Key Laboratory of Physical Chemistry of Solid Surfaces,College of Chemistry and Chemical Engineering, Xiamen University, 361005, Xiamen, China
Tel: +86-592-2185905 (Lab) Fax: +86-592-2183905 Email: qfdong@xmu.edu.cn, mszheng@xmu.edu.cn
